How Does an Electron Become Excited
An electron can become excited if it is given extra energy such as if it absorbs a photon or packet of light or collides with a nearby atom or particle. Since changing direction is acceleration oscillations produce radiation.
A light source with the appropriate wavelength raises the electrons in the fluorochrome to a higher energy electronic orbital.
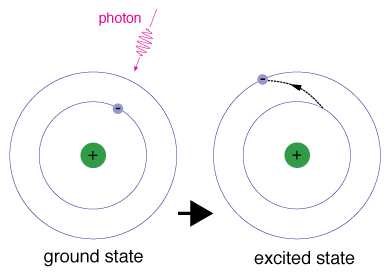
. It means the electron has more energy than its ground state energy. An electron can become excited if it is given extra energy such as if it absorbs a photon or packet of light or collides with a nearby atom or particle. Electrons that are bound by some central potential say bound to an atomic nucleus for example have individual energy levels that they can inhabit.
The electron energy involved is due to the potential energy well from attraction to the positive charge of the nucleus. For an electron to be boosted to an orbital with a higher energy it must overcome the difference in energy between the. These are called excited states of the atom.
Light excites an electron in a pigment found in the light harvesting complexes of the photosystem rank the electrons that occur in photsystem II in order of potentail energy levels beginning with the highest potential energy. 1s2 2s2 2p3 3s1 - where the valence electron now occupies the 3s orbital in an excited ie. A photon of light energy travels until it reaches a molecule of chlorophyll.
When an electron absorbs energy it will move up from a lower energy level to a higher energy level called the excited state of the negatively-charged subatomic particle. When given energy electrons move to a higher energy level known as an excited state. If the photon has too much energy the electron will cease to be bound to the atom and the atom will become ionized.
AstoundingJB The further the electron is from the nucleus the slower the. The photon causes an electron in the chlorophyll to become excited The energy given to the electron allows it to break free from an atom of the chlorophyll molecule. Chlorophyll is therefore said to donate an electron Figure 512.
The electron will move from a higher PEL to a lower PEL as it returns to ground state. How does an atom become excited. The lowest possible energy level is the ground state.
While the electron is in the higher state usually 1 to 10 ns it can undergo non-radiative transitions in which the energy is dissipated as heat vibrations to the solvent. Electrons become excited when they absorb energy. Light Energy Each orbital has a specific energy associated with it.
This happens through some energy absorbtion whether that be heat electricity or light. It is important to note that anytime you accelerate a charged particle it will produce EM radiation. When you shake a charge back and forth it causes the EM field to oscillate as well.
Atoms become excited when electrons go from the ground state to an orbital level requiring more energy to exist there. The energy of the emitted radiation equals the energy that was originally absorbed by the electron minus other small quantities of energy lost through a number of secondary. When an electron temporarily occupies an energy state greater than its ground state it is in an excited state.
These oscillations generate photons aka EM radiation. How does an electron become excited. By giving the atom additional energy for example by the absorption of a photon of an appropriate energy the electron is able to move into an excited state one with one or more quantum numbers greater than the minimum possible.
Mar 13 2014 at 1443. So for oxygen it might look like this. In an atom electrons prefer to stay in the orbitals closest to protons known as the ground state.
Light is emitted given off when an excited electron releases energy and returns to its original ground state. If the element were to become excited the electron could occupy an infinite number of orbitals. Answer 1 of 3.
An electron can become excited if it is given extra energy such as if it absorbs a photon or packet of light or collides with a. The electrons of an atom are able to absorb particles of light called. However the absorbed energy is released within a small interval of time and the electron moves down to its ground state.
In these states the energy of the electrons and the atom on the whole is higher. Hydrogen Bright Line Emission Spectrum. When I was a kid I would go camping a.
Eventually the excited electron loses the extra energy by emitting electromagnetic radiation of lower energy and in doing so falls back into its original and stable energy level. The electron is not stable at all levels in the potential energy well. How do electrons become excited.
According to Bohrs theories the electrons in the metal ions areexcited due to the absorption of a quantum or multiple quanta oflight. When an electron temporarily occupies an energy state greater than its ground state it is in an excited state. Also there exist some other states where the electrons revolve faster or farther from the nucleus.
However in most texts the example will be the next available one.
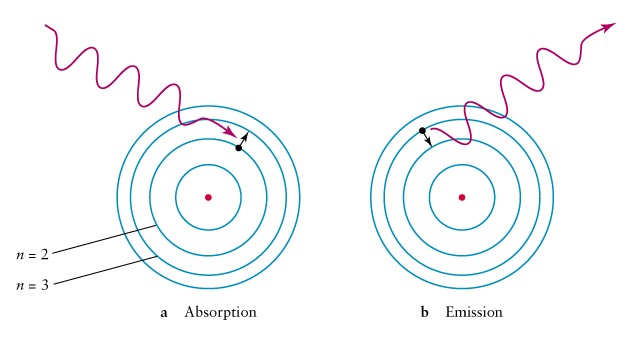
When An Electron Goes From Ground State To Excited State Does It Absorb Or Lose Energy Socratic
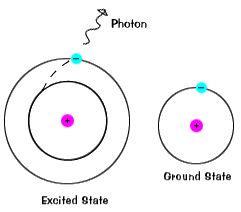
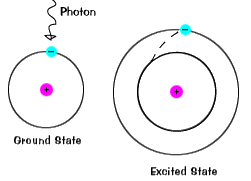
Belum ada Komentar untuk "How Does an Electron Become Excited"
Posting Komentar